Oracle APEX enabled PharmaLex to automate the distribution of safety documents and improve regulatory management in the pharmaceutical and medical device industries.
Business challenges
PharmaLex, part of Cencora, specializes in providing solutions for the pharmaceutical, biotechnology, and medical technology industries. The company was building a SaaS solution, psiXchange, to help its customers overcome common operational and compliance challenges in managing the distribution of clinical trial safety reports. The traditional, manual safety document submission process relied on the use of spreadsheets and maintained lists of recipients, which not only increased the risk of errors but also required significant effort and resources. In addition, the increasing volume and complexity of safety reports made the previous system ineffective.
PharmaLex identified the need for a more efficient solution that would not only streamline the document distribution process but also comply with stringent industry regulations. As a result, the company sought a coding platform that would help improve accuracy, speed, and compliance. By choosing Oracle APEX, PharmaLex was able to deliver an automated document distribution solution to its customers, improving efficiency, reducing manual effort, and increasing the quality and auditability of the process.
“I joined the Oracle APEX development team early on. It’s great to see PharmaLex products grow to production-ready. We’ve always found APEX to be a great, cost-effective low-code solution for quickly building great-looking, scalable applications”.
– Roel Hartman , APEX Developer for psiXchange and psiQ.
Why did PharmaLex choose Oracle APEX?
Seeking a more efficient coding solution, PharmaLex chose Oracle APEX because of its strong previous experience with Oracle technology and its ability to leverage existing PL/SQL code. The company had good experience with previous versions of APEX, which eased the transition and reduced development risks.
APEX stood out from the competition for its ability to continuously adapt, enabling rapid development of scalable and visually appealing applications. This low-code solution not only optimizes the speed of document delivery but also enhances regulatory compliance monitoring, a critical aspect in the pharmaceutical industry.
PharmaLex valued Oracle’s technical support and attention to detail, which ensured an agile and effective implementation. In addition, Oracle’s price competitiveness was crucial in optimizing costs without compromising quality or functionality, enabling Cencora and PharmaLex to strengthen their position in a complex regulatory environment.
Results
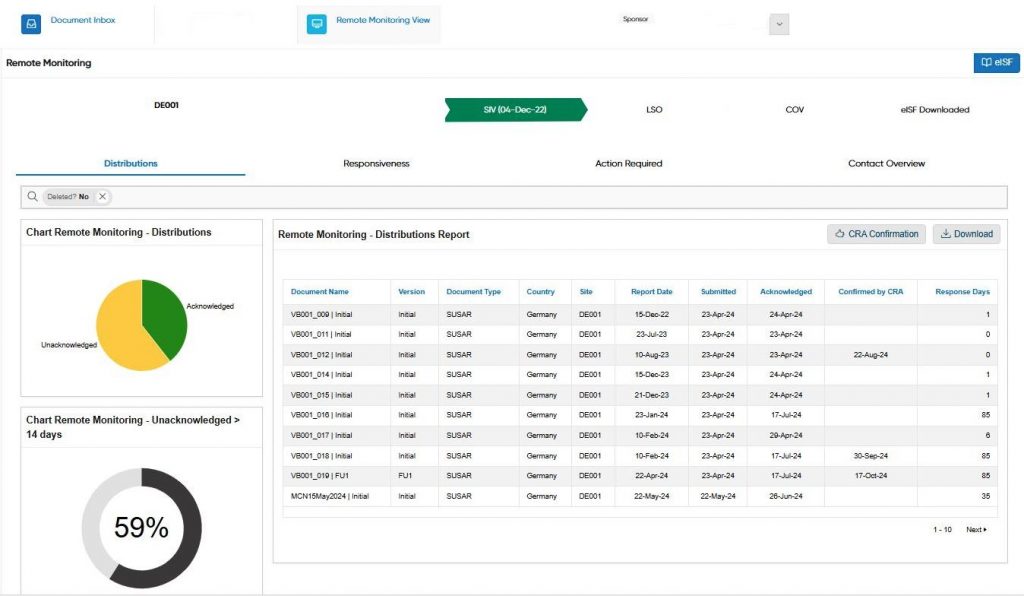
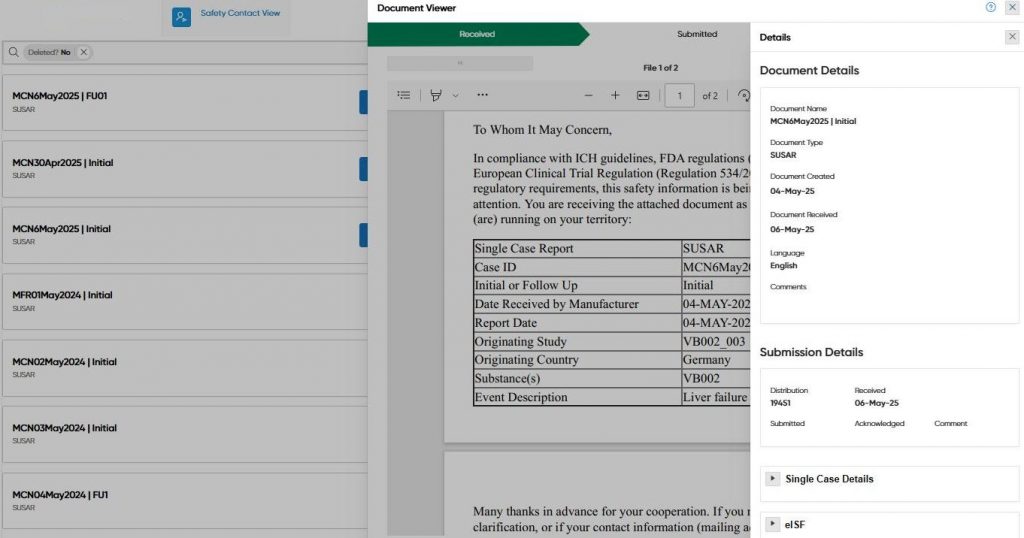
The implementation of Oracle APEX has provided PharmaLex with significant benefits in developing an innovative solution to improve safety document management in the pharmaceutical industry. Built on APEX, the company’s psiXchange SaaS software has achieved up to a 95% site response rate (system data) and up to a 90% reduction in resource requirements. These improvements optimized internal processes, increased efficiency, and reduced the risk of compliance errors. Customers gain the confidence that the right safety reports are delivered to the right people at the right time.
Moreover, APEX capabilities have helped psiXchange enable better collaboration between teams. Users have easy access to critical information, allowing them to focus on more strategic tasks, increasing satisfaction and productivity.
Another important benefit is the scalability that APEX offers. PharmaLex tools such as psiMonitor enable the centralized monitoring of applications, the efficient management of multiple instances, and a rapid response to errors, ensuring continuous compliance with safety regulations.
The development of the complete suite of applications, including psiQ, psiXchange, psiCentral, psiMigration, and psiMonitor, was achieved through an ongoing development process using APEX. In addition, PharmaLex implemented an optimized CI/CD process using SQLcl with Liquibase and Gitlab, allowing them to efficiently deploy application releases across multiple environments.
Oracle APEX has also enabled PharmaLex to quickly adapt to market demands and improve its time to market. The combination of these quantitative and qualitative benefits confirms the effectiveness of Oracle APEX as a key solution for optimizing safety document management, increasing operational efficiency, and ensuring high levels of compliance in the pharmaceutical industry.


About the customer

PharmaLex is part of Cencora, a leading global pharmaceutical solutions organization centered on improving lives around the world. PharmaLex adds to Cencora’s expanding suite of pharma solutions and serves the pharma, biotech, and medtech industries. The company guides clients from early strategic planning activities and non-clinical requirements through clinical development, regulatory submission processes and post-approval / maintenance post-launch activities. PharmaLex experts use technology-elevated solutions to support clients through the entire product lifecycle.
Product list: Oracle APEX, Oracle Argus, Oracle Cloud Infrastructure (OCI), SQL Developer, REST Data Services

